Bringing Optical System Manufacturing into Focus
As a contract manufacturer of electronics-based medical devices and life sciences instruments, our infrastructure has been specifically designed to support the full range of services required to develop and build complex mechatronic systems, with optics being one of our key focuses. Our optical capabilities involve integrating, testing and aligning optical components – such as lenses, mirrors, sensors, optical switches, cameras, illuminators, dichroic filters and optical analyzers – into the design and manufacture of a device. The purpose of optics in medical devices varies, but common applications include DNA scanning, cancer cell imaging, surgical analysis, medical video systems, light therapy and laser-based procedures. Manufacturing of such medical devices requires precision, attention to detail and a high level of technical expertise to ensure that products meet the industry’s stringent quality and reliability standards. It is therefore important to make sure that your contract manufacturing partner has all the necessary facilities, equipment and procedures in place to mitigate risks and deliver consistent results.
Controlling Contamination
Healthcare instruments often contain extremely sensitive optical components and, without suitable equipment and strict process controls in place, particulate contamination can occur during production, leading to manufacturing defects and costly rework. Our 4,000 square foot optical assembly room, constructed specially to control contamination, allows no more than 1,000 particles larger than 0.5 microns to be present per cubic foot of air. The facility contains a full suite of scalable optical-alignment workstations featuring our innovative vPoke® technology, a zero-defect, computer-directed process that offers significant improvements in assembly, testing and alignment efficiencies, as well as increased quality control and traceability. Several practices and protocols must be followed in order for the optical assembly room to effectively maintain the level of cleanliness required to build optical systems, including:
- Controlled access: only authorized personnel are allowed to enter, and they must follow proper gowning procedures to prevent the introduction of particles.
- Positive air pressure: the optical assembly room is maintained at a positive air pressure relative to the surrounding environment to prevent the ingress of particles.
- HEPA filtration: high efficiency particulate air filters are used to capture particulates from the air before it is circulated back into the room.
- Controlled environment: the temperature and humidity levels are controlled to minimize the generation of particles.
- Minimal surface contamination: all surfaces are cleaned regularly to avoid contamination.
By following these protocols, the optical assembly room serves as a controlled environment for optical system builds, minimizing the risk of particulate contamination and ensuring the reliability and quality of the finished product.
Planning for Success
Our dedicated team of certified optical engineers and technicians will work closely with you prior to manufacturing to compile a mutually agreed upon project charter. This outlines all optical handling, assembly and inspection procedures in detail, and provides a set of templates and checklists covering every aspect of process validation, system build qualification, and design output and verification. Such rigorous testing protocols are essential to verify that a finished product meets the required performance specifications.
OPTICAL DESIGN OUTPUT REQUIREMENTS
- Layout (schematic, beam propagation description)
- GUI or script to run the optical system
- Drawings of optical subassemblies
- Part handling/cleaning requirements (mirror, lens, dichroic filter, beam splitter, harmonic generator, etc.)
- Gluing requirements (adhesive, cure time)
- Mechanical and hardware cleaning requirements (ultrasonic, vacuum oven, solvents)
- Laser and system burn-in requirements
- Assembly method sheets
- Optical alignment procedures (alignment, test)
- Optical alignment test fixture (target block, alignment tool, light source, energy meter, power control, beam view, etc.)
- Final test reports
OPTICAL DESIGN AND PROCESS VALIDATION REQUIREMENTS
- IQ/OQ/PQ validation for all test fixtures before launching any product into production.
- Spec verification for the optical system (laser power output, beam size, beam divergence, beam quality, beam pointing, M2, final power output, stability, satellite spec, laser leakage)
- Optical packaging requirement
- Environmental testing (results for vibration, drop, pointing stability, lifetime, room temperature requirement, humidity, EMI)
- Test data/results from alpha and beta build
- Troubleshooting guide
- Biological test vs. optical alignment issue list
End-to-End Quality Assurance
It’s important when you’re choosing your contract manufacturer to look for a partner who has the necessary infrastructure and process controls to support your specific project needs, as well as a proven track record in the medical device and life sciences markets. Paramit is a reliable and trusted OEM partner that specializes in the manufacture of instrumentation that requires top-grade optical assembly builds and integration. Our complete end-to-end optical service – including new product introduction, design for manufacturing, failure analysis and real-time manufacturing insights – covers the entire product realization life cycle to ensure a quality product and a faster time to market.
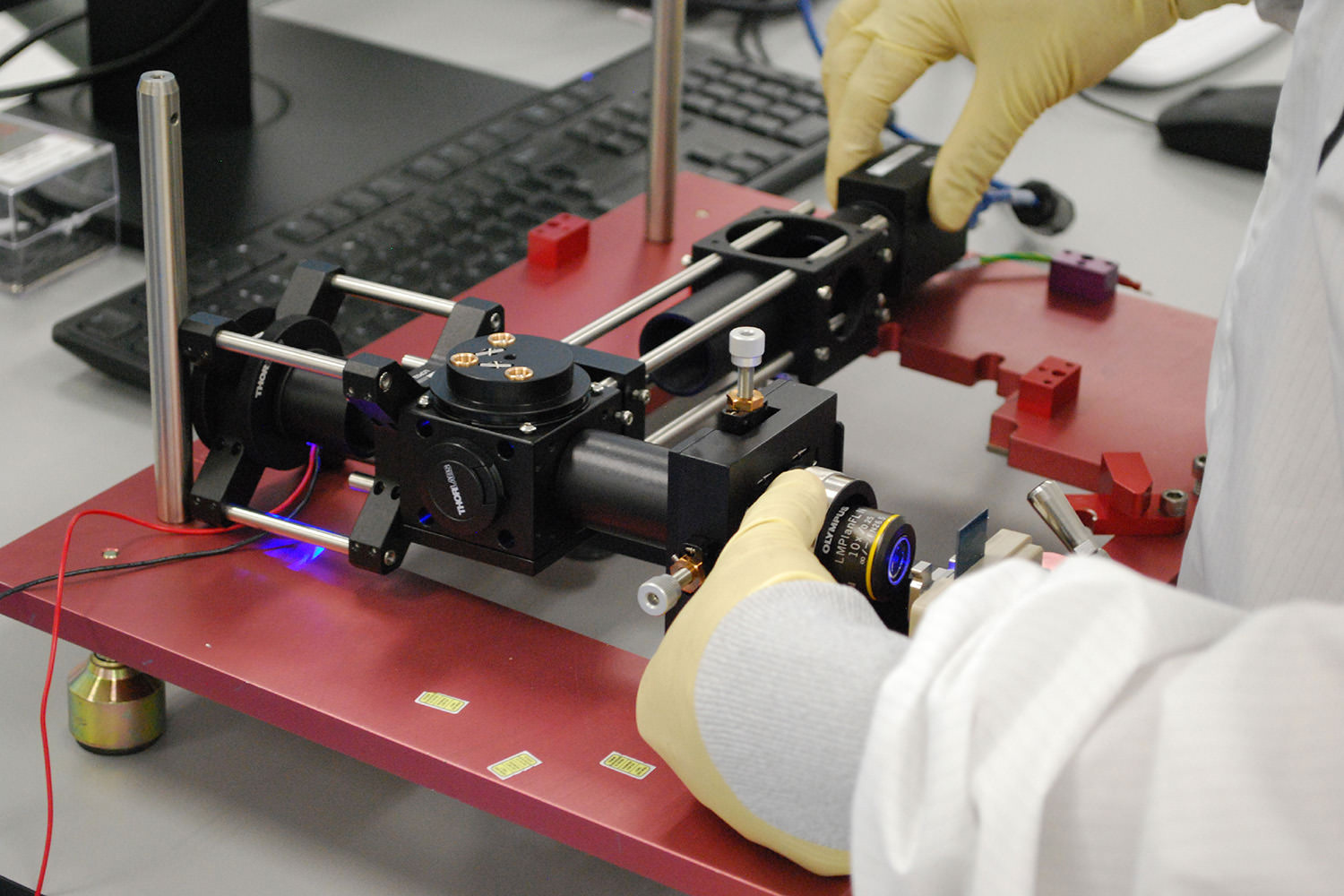
Optics technicians working.
Optics technicians working on optical subassemblies.
Paramit’s optical assembly room.